US Guided Thoracentesis | Paracentesis
In this chapter we will take a look at the use of ultrasound guided thoracentesis and paracentesis. This chapter assumes you know how to use ultrasound of the lung for diagnostic purposes so head out here in case you may have missed it. Some of the equipment and steps are similar for both procedures but we will deal with them separately in this section.

US Guided Thoracenthesis
Thoracentesis is the removal of pleural fluid from the pleural space in the thoracic cavity and can be done for either diagnostic or therapeutic reasons. Ultrasound has several clinical implications when used to guide the procedure including:
1. Ultrasound (US) can be used to identify and guide the percutaneous removal of pleural fluid. The technology allows identification of small amounts fluid (<30ml) that cannot be identified with the use of radiography which typically detects volumes in excess of 50ml of fluid. Ultrasound can also identify loculations more readily than with radiography.
2. US should be used to guide thoracentesis to reduce the risk of complications, the most common being pneumothorax. It does this by identifying patients in whom thoracentesis cannot be safely performed, allowing selection of the safest needle insertion site and revealing the optimal depth of needle insertion.
3. US should be used to increase the success rate of thoracentesis. It's use has lower rates of failed attempts.
Contraindications to thoracentesis include severe bleeding diathesis, skin infection or wound over the needle insertion site.

Anatomic considerations Thoracentesis
The space between the parietal and visceral pleura is the pleural space and the location of pleural effusions. It is this space that we need to access with thoracentesis. The pleural space extends inferiorly to approximately the 10th intercostal space. The intercostal neurovascular bundle runs along the inferior border of the rib (as shown in the diagram to the right) and should be avoided when performing the thoracentesis.
Lung
Internal intercostal muscle
Parietal Pleura
Visceral Pleura
Diagram showing a section of an intercostal space and relevant structures.
Safe limits for thoracentesis

A safe region for thoracentesis is the area superior to the 9th rib (due to diaphragmatic excursion), 5cm lateral to the spine and posterior to the mid axillary line (area shown on the picture to the left).
Safe region for thoracentesis. See text

Equipment and Technique
To locate the entry point of the needle for thoracentesis a low frequency (1-5 Mhz) probe work best by allowing us to look at deeper structures. A curved linear and a cardiac (phased array) probe would work best for this. The advantage of the curved linear probe would be that the lung sliding would be easier to visualize than with a cardiac probe. However, it has a larger probe footprint and thus real time guidance needle guidance may be challenging to perform with this probe.
A linear probe is useful if the procedure is intended to be done using real time ultasound.
Example of low frequency probes that can be used for thoracentesis. Once these probes have been initially used to confirm potential needle locations for drainage, a linear probe can then be used if real time ultrasound is warranted.
Probe selection
Thoracentesis equipment
The provider should have the necessary equipment before attempting thoracentesis. Several commercially available kits are available that contain items needed for the thoracentesis (see picture below). Most kits contain a 8-Fr over-the-needle catheter (for therapeutic purposes), an 18-gauge needle (for diagnostic purposes), a stopcock and a 35 to 60ml syringe with a thoracentesis drainage system. You may need an ultrasound probe cover with sterile gel since it is typically not included in the kit.

8 F over an 18G Needle
18 G Needle
An example of a thoracentesis kit. This is the Arrow-Clarke Pleural Seal Kit.

Thoracentesis steps summary.
The following is a summary of the steps to guide you in performing thoracentesis after positioning the patient sitting upright on the edge of the bed with arms extended. :
2. Sterile prep and drape. Follow full barrier precautions and prepare the surgical area is prepped with chlorhexidine (if not allergic) for 60 seconds. The ultrasound probe can also be used to guide the needle as it goes into the thorax. If this is the case then the probe should also be covered with a sterile cover.
3. Apply local anesthesia. The proposed entry site from step 1 should have resulted in an indentation of the skin by firmly pressing the skin. Local anesthesia should be given at the level of the skin by creating a skin wheel at this site.
1. Pre procedural scanning. We start by evaluating and mark the location of the site of insertion before an actual attempt is made with the use of ultrasound. This critical step ensures the correct side and amount of fluid that is meant to be sampled or collected. It also ensures that other organs have been accounted for and out of the way of the needle pathway. Remember the angle of approach, which is the angle between the probe and the skin. The thoracentesis site should be in the mid scapular or posterior axillary line and one or two intercostal levels below the highest level of effusion. We will be exploring this step further in the section on sonoanatomy.

Pre Procedural scanning.
4. Needle insertion. In order to minimize complications and to gain access to the thoracic cavity a special consideration must be made regarding the location of the entry site. The provider needs to avoid the neurovascular bundle that runs on the underside (below the rib) of the interspace being punctured (seen on the image). The needle is advanced while performing negative aspiration on the plunger and is advanced until there is fluid on the syringe or loss of resistance. The recommendation here is to use the same angle of approach as that used with the probe during the pre procedural phase. The providers will have two options here:
4i. If the effusion is deemed large enough the provider may continue with the procedure without the additional use of ultrasound.
4ii. If the effusion is small or confirmation of the target space is needed then real time ultrasound is use to guide the needle into the thoracic cavity.
Diagram showing a section of an intercostal space and a pleural effusion. Notice the insertion site of the needle at the intercostal space.
5. Advance catheter. Negative aspiration has produced pleural fluid coming into the syringe as a results of the steps above. We can now collect fluid for sampling. If using a catheter over the needle then it is advanced and secured and then have the collection bag placed.
6. Confirm normal lung sliding with Ultrasound. Confirmation of normal lung sliding can be used to rule out pneumothorax pre and postprocedure. Post procedure chest radiography may be considered in those patients who are mechanically ventilated especially if they present with high airway pressures.
Right diaphragm and intra-abdominal organs.
SCM
In these clips we see a side by side comparison of the windows we obtain by moving the ultrasound probe from the right lower back moving upwards in order to identify the diaphragm. On the right side we should see the liver and the right kidney and as we move the probe more cephalad, the diaphragm appears. We can also appreciate the acoustic shadows cast by the ribs that obscure the visualization of deeper structures.

1
2

1
2
D
L
K
S
S
Right lower chest. Movement of the probe cephalad (from position 1 to position 2 which lies more posterior and higher up) and corresponding windows on the right. LS, lung sliding; L, liver; D, diaphragm; K, kidney. Notice the curtain effect which is an artifact seen when the diaphragm contracts which then lowers the lung into the US window and since there is normal lung sliding, this will in turn obscure deeper structures including the diaphragm.
Right diaphragm and intraabdominal organs.
In these clips we see a side by side comparison of the windows we obtain by moving the ultrasound probe from the left lower back moving upwards in order to identify the diaphragm. On the left side we should see the spleen and the left kidney and as we move the probe more cephalad, the diaphragm appears. We can also appreciate the acoustic shadows cast by the ribs that obscure the visualization of deeper structures. Notice that we see a curtain effect on this side and we cannot visualize the diaphragm directly. This is because at ultrasound beam has encountered lung sliding more proximal to the probe and thus sound waves are not able to penetrate deeper into the tissue.

1
2

1
2
LS
K
S
Left lower chest. Movement of the probe cephalad (from position 1 to position 2 ) and corresponding windows on the right. LS, lung sliding; S, spleen; K, kidney. Notice the curtain effect obscures the direct visualization of the diaphragm.
Posterior chest wall.
As we move the probe more cephalad we notice that the intra-abdominal structures are now out of view. We can see normal lung sliding and the presence of B lines on this clip.

3
1
S

LS

3
Movement of the probe more cephalad. We appreciate absence of the intra abdominal organs. We can also see lung sliding (LS) implying that the parietal and visceral pleura are in contact with each other. We also appreciate B lines on this clip.

Pleural Effusion on US
US can be used to approximate the volume of pleural fluid to guide clinical decision-making. A semiquantitative estimation of pleural fluid volume can be made on US.
The British Thoracic Society Pleural Disease guidelines recommend at least 10mm of pleural fluid between the parietal and visceral pleura. A pleural effusion spanning more than 3 intercostal spaces by US corresponds to a volume greater than 1L of fluid.

Pleural effusion. A large anechoic collection of fluid can be seen above the diaphragm. This represents a simple pleural effusion seen. Heparization of lung and Spine sign are seen in this clip. This lung appears to be floating freely the pleural fluid. The sonographer should lower the depth move the ultrasound higher into the chest in order to avoid the diaphragm.
Ultrasound can be used to measure the depth from the skin to the parietal pleura to determine appropriate length of the needle and determine its maximum needle depth.

Simple pleural effusion. A large anechoic collection of fluid can be seen above the diaphragm. We can appreciate a significant distance from the thoracic wall to the lung parenchyma in this location.
If the size of the pleural effusion is deemed small or the provider would like to see the needle going in the space in real time, a linear probe is then used. This probe must be draped with a sterile technique and a longitudinal scan is used on the interspace selected. An off plane approach is then used visualize the needle in the center of the ultrasound machine. Other approaches have been described including the use of a curved linear probe and in an in-plane approach.

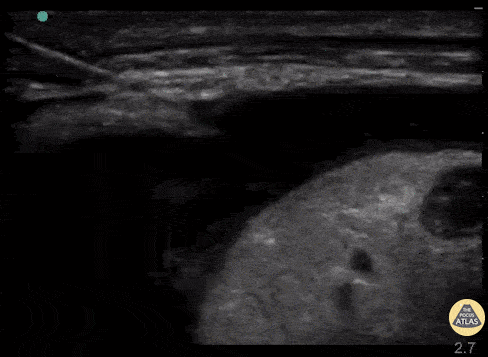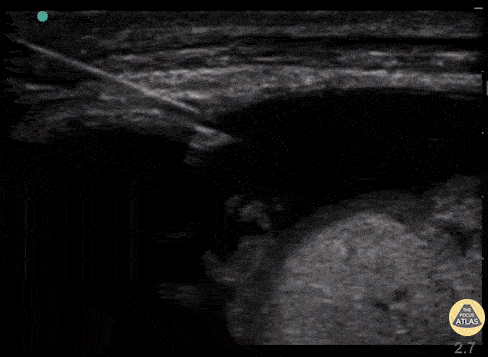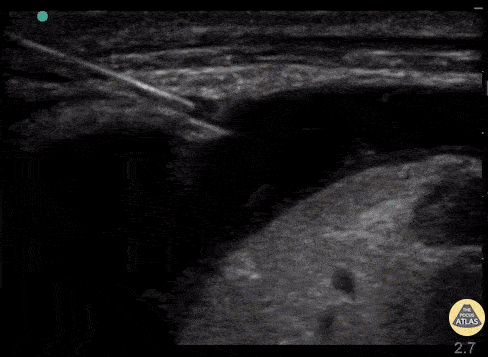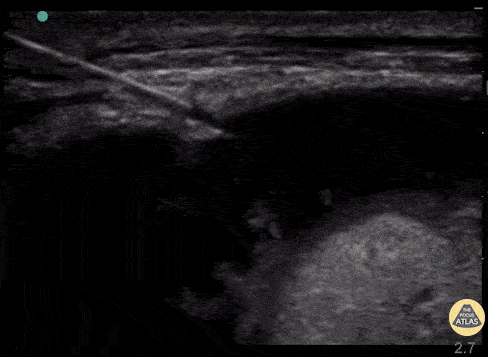
Real time US for thoracentesis. Linear probe in the longitudinal plane and thoracentesis needle approaching off plane. The needle is visualized as it transverses the layers into the pleural space while the provider performs negative aspiration. Ultrasound image courtesy of thepocusatlas.com
Pleural effusions can be categorized as simple or complex sonographically. Simple effusions are anechoic and most often transudative. Complex effusions are those with fibrin strandings and septations. Expert consultation may be necessary when attempting to drain these.


Types of pleural effusions. On the left a simple effusion characterized by anechoic fluid. On the right we appreciate septations that are typical of complex effusions. Right clip courtesy of The Pocus Atlas.
Lung Consolidation
US better differentiates effusions from consolidations compared to chest radiography. A semiquantitative estimation of pleural fluid volume can be made on US.A lung that has consolidation is a lung that is dense and allows us to see it under US. The lung appears with equal echogenicity as that of the liver and thus referred to as hepatization of the lung. If there bronchial structures that supply the affected lung are patent, there may be air bronchograms within it. All causes of infiltrative process or severe pneumonia with complete filling of the alveolar compartment will yield complete filling of the alveolar compartment.

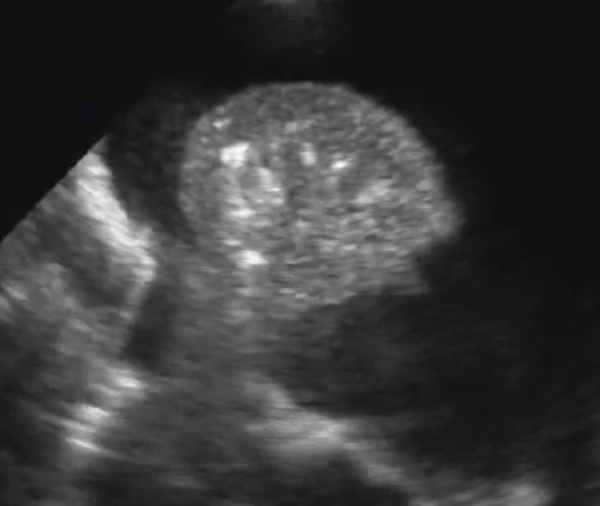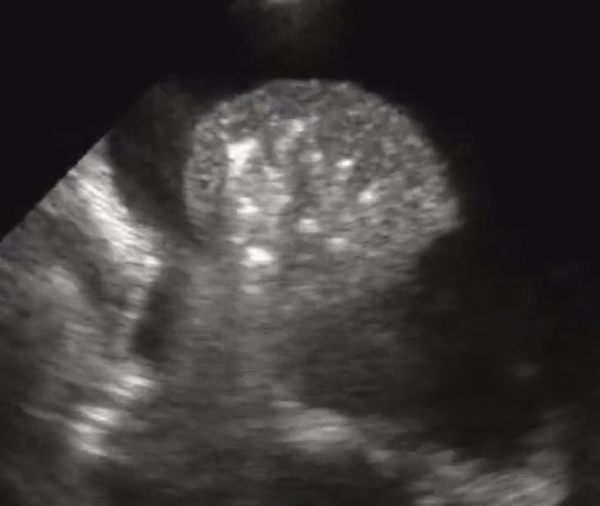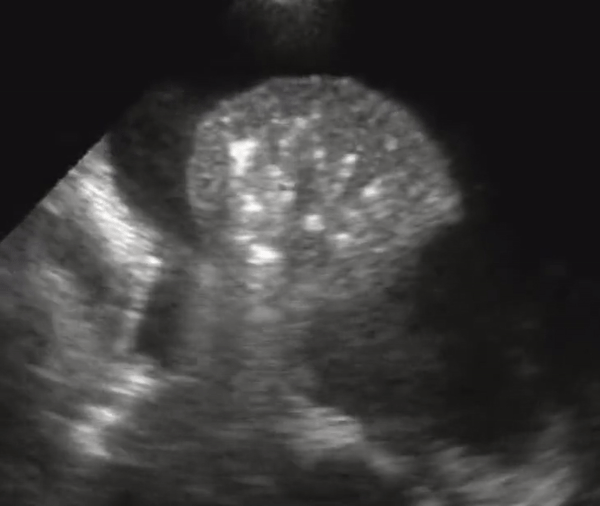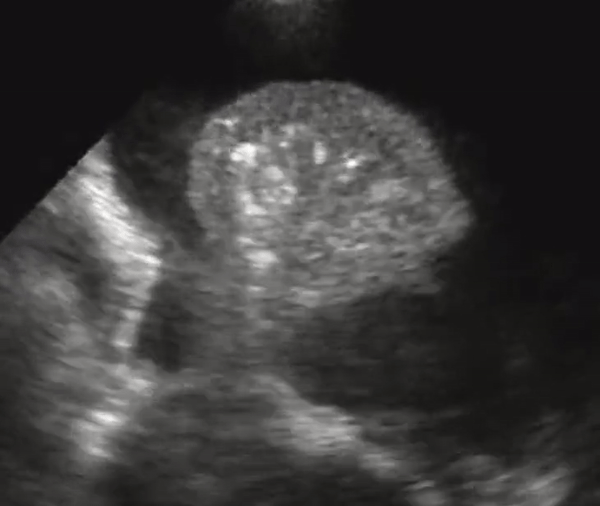
Consolidation with complete filling of the alveolar compartment. Air bronchograms appear as hyperechoic (white) structures on the lung parenchyma. There is a small amount of pleural effusion. On the right we also appreciate pleural effusion.

Ultrasound Guided Paracentesis
Clinical indications for paracentesis include suspicion of spontaneous bacterial peritonitis, evaluation of the etiology of ascites and to relieve dyspnea or discomfort from large volume ascites.
US is recommended for similar reasons than those for thoracentesis and they include:
1. It's use reduced the risk of serious complications. The most common complication being bleeding.
2. US can be used to identify those who can benefit form fluid drainage. It can be used to assess if there is enough intraperitoneal fluid that can be drained. Abdominal physical examination is not a reliable method for determining the presence or volume of intraperitoneal free fluid. US can detect as little as 100ml of peritoneal free fluid.
3. US can be used to assess volume and location of intraperitoneal free fluid and determine safe puncture site. US improves overall success rate by locating the largest fluid collection and guiding the location of the needle puncture site.
Contraindications to paracentesis include a surgical abdomen. Relative contraindications include severe bleeding diathesis, pregnancy, intraabdominal adhesions, skin infection or wound over the needle insertion site. Complications from paracentesis include hypovolemic shock, bleeding, perforation of visceral organs and peritonitis.
Now that we have stablished the usefulness of US lets take a look at the anatomic considerations, steps and sonographic appearance of paracentesis.

Anatomic considerations for Paracentesis
The peritoneal cavity normally contains approximately 50-75 mls of fluid to lubricate the tissues that lie the abdominal wall and viscera Ascites is thus an abnormal accumulation of fluid. This fluid typically accumulates in the most dependent portions of the abdominal cavity and in the supine position this would correspond to Morrison's pouch (hepato-renal fossa) which is why patients need to be in a semi-recumbent position when performing the procedure.
The liver and spleen make the upper quadrants and thus these sites will not be potential targets of paracentesis. Also the bladder must by empty before an attempt is made.
It is important to recognize the vessels that run through the abdominal wall since bleeding is the most common complication from paracentesis. The superficial epigastric artery (SEA) is a branch of the femoral artery that arises below the inguinal ligament and runs between the two layers of the superficial fascia anterior to the rectus abdominis muscles up to the level of the umbilicus. The inferior epigastric artery (IEA)is a branch of the external iliac artery runs deep into the rectus abdominis muscle to later anastomose the superior epigastric artery. Finally the deep circumflex iliac artery runs lateral (DCI) is also a branch of the external iliac artery and ascends posterior to the inguinal ligament that travels to the anterior iliac spine and then ascends between the internal oblique and transversus muscle. The anatomy of the superficial vessels varies greatly however and it is a reason to perform Color Flow Doppler (CFD) to identify them along the plan needle trajectory.


SEA
IEA
DCI
L
Perfusion of the abdominal wall. The superficial epigastric artery (SEA) runs between the two layers of the superficial fascia anterior to the rectus abdominis muscles up to the level of the umbilicus. The inferior epigastric artery (IEA) runs deep into the rectus abdominis muscle. The deep circumflex iliac artery runs lateral (DCI) ascends between the internal oblique and transversus muscle.
It is also important to recognize the layers of muscle we need to penetrate in order to have access to the parietal peritoneum depending on the planned approach and the close proximity of abdominal wall to the peritoneal organs.

RAM
EOM
IOM
TAM
PP
Cross section of abdomen. Rectus abdominis muscle, RAM; external oblique muscle, EOM; internal oblique muscle, IOM; transversus abdominis muscle (TAM); parietal peritoneum (PP). Image courtesy of The Human Project

Equipment and Technique
To locate the entry point of the needle for thoracentesis a low frequency (1-5 Mhz) probe work best by allowing us to look at deeper structures. A curved linear and a cardiac (phased array) probe would work best for this. The curved linear probe is optimal if the consideration is to do the procedure with US in real time.
Probe selection
Example of low frequency probes that can be used for thoracentesis. Once these probes have been initially used to confirm potential needle locations for drainage.
Paracentesis equipment
The provider should have the necessary equipment before attempting paracentesis. Several commercially available kits are available that contain items needed for the thoracentesis/paracentesis (see picture below). Most kits contain a 8-Fr over-the-needle catheter (for therapeutic purposes), an 18-gauge needle (for diagnostic purposes), a stopcock and a 35 to 60ml syringe with a thoracentesis drainage system. You may need an ultrasound probe cover with sterile gel since it is typically not included in the kit.

8 F over an 18G Needle
18 G Needle
An example of a thoracentesis/paracentesis kit. This is the Arrow-Clarke Pleural Seal Kit.

Paracentesis summary.
The following is a summary of the steps to guide you in performing paracentesis after positioning the patient in a semirecumbent position:
2. Sterile prep and drape. Follow full barrier precautions and prepare the surgical area is prepped with chlorhexidine (if not allergic) for 60 seconds. The ultrasound probe can also be used to guide the needle as it goes into the abdomen (real time use of US). If this is the case then the probe should also be covered with a sterile cover.
3. Apply local anesthesia. The proposed entry site from step 1 should have resulted in an indentation of the skin by firmly pressing the skin. Local anesthesia should be given at the level of the skin by creating a skin wheel at this site.
1. Pre procedural scanning. We start by evaluating and mark the location of the site of insertion before an actual attempt is made with the use of ultrasound. This critical step ensures the correct side and amount of fluid that is meant to be sampled or collected. It also ensures that other organs have been accounted for and out of the way of the needle pathway. Remember the angle of approach, which is the angle between the probe and the skin. Scanning should be on multiple orientations (transverse and sagittal for example) to visualize intraperitoneal organs. Turn on Color Flow Doppler (CFD) to assess for vasculature along the planned trajectory site.
Pre Procedural scanning. See below for sonographic imaging.

4. Needle insertion. In order to minimize complications and to gain access to the abdominal cavity a special consideration must be made regarding the location of the entry site. The needle is advanced while performing negative aspiration on the plunger and is advanced until there is fluid on the syringe or loss of resistance. The recommendation here is to use the same angle of approach as that used with the probe during the pre procedural phase. The providers will have two options here: To use the marked location or to use the ultrasound probe in real time with an inline approach. The patient should remain in the same position between marking of the site and needle insertion if US is not being used in real time.
4i. If the ascites is considered to be small or confirmation of the target space is needed then real time ultrasound is use to guide the needle into the abdominal cavity. Orthogonal views are recommended before proceeding to confirm deeper structures are away from the planned trajectory site.

In line, real time US use for paracentesis. See below for sonographic imaging.
5. Advance catheter. Negative aspiration has produced pleural fluid coming into the syringe as a results of the steps above. We can now collect fluid for sampling. If using a catheter over the needle then it is advanced and secured and then have the collection bag placed.

Sonographic Anatomy Abdominal Wall
Lets have a look at what US images without ascites to appreciate the proximity of abdominal wall muscles and the peritoneal cavity. The parietal peritoneum and transverse fascia appear as a hyperechoic line that separates the abdominal wall from the peritoneum.

AA
AV
PMi
PMa
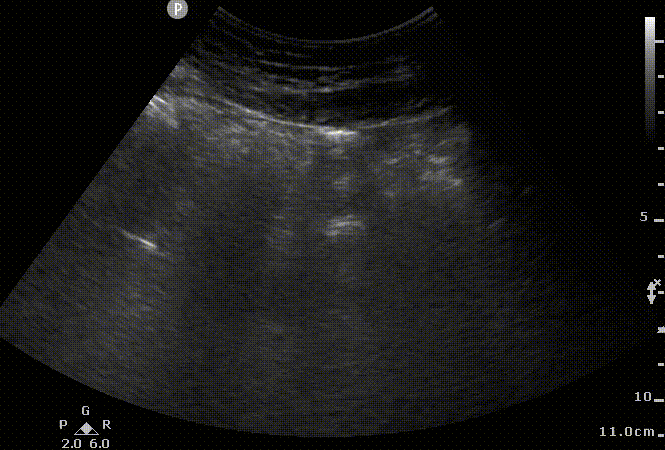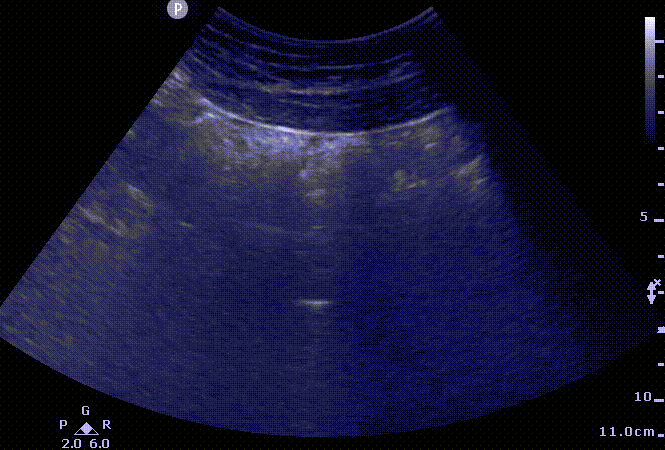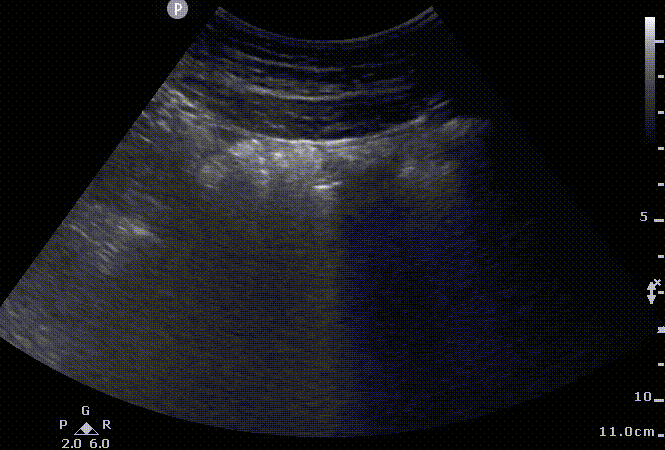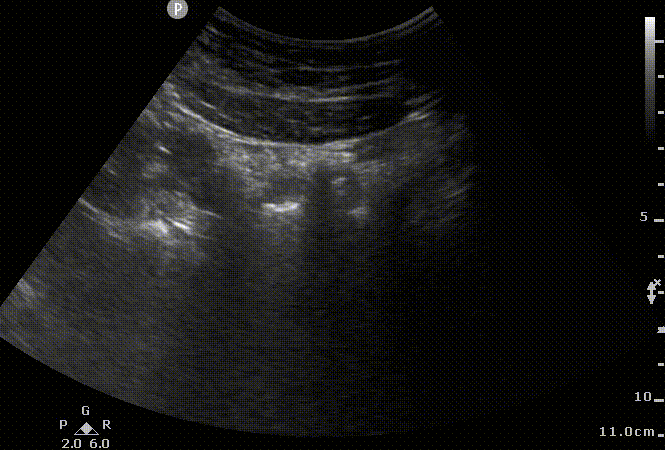
AWM
PP
L
Sonographic appearance of abdominal wall without ascites. We can appreciate small movement on the lateral wall as a curved linear probe is moved down in a transverse plane and the corresponding 2D US images. Abdominal wall muscles (AWM); peritoneum (PP), Bowel (B). Notice the acoustic shadows cast by the air encountered in the loops of the bowel.
Sonographic Evaluation of Ascites.
Ascites will typically appear dark on ultrasound (anechoic) and most pronounced collections of fluid will typically correspond to the most dependent area of the abdomen. Loops of bowel with mesentery will appear floating in this fluid and move with respiration. Hemoperitoneum will produce a complex appearance of ascites fluid with internal echoes of blood interspaces with fluid. Loculations caused by fibrin standing may also be present.
The optimal needle insertion site is best determined based on a combination of visualization of the largest fluid collection, avoidance of underlying organs and the thickness of the abdominal wall. A measurement of the wall thickness (from the skin) will give an idea of the distance the needle has to penetrate in order to reach the peritoneum and the optimal length of the needle required. A patient who has abdominal wall edema or a thick subcutaneous tissue will have a higher distance and a longer needle may be necessary. We can thus evaluate an 'Ascites Safety Zone': the ascites filled space that lies between the peritoneal lining and the bowel.


Sonographic view of ascites. Simple ascites visualized. Anechoic fluid is visualized with this curved-linear probe and most likely corresponds to transudate. Notice the acoustic enhancement created by the presence of fluid in the abdomen. The ascites safety zone (line in blue) on this clip corresponds to approximately 3 cm. So the needle will have to penetrate the muscular wall (in purple) and then have the safety zone before injuring bowel wall in this location.
The needle insertion site should be evaluated in multiple planes to ensure avoidance of underlying abdominal organs and detect blood vessels along the planned needle trajectory. Color Flow Doppler should be used to assess for superficial vessels along the anticipated needle trajectory (not shown on the clips below).


Sonographic view of ascites. This view is orthogonal (90 degrees) from the above clip. Anechoic fluid is visualized with this curved-linear probe and most likely corresponds to transudate. Notice the acoustic enhancement created by the presence of fluid in the abdomen. The safety margin looks higher on this one (blue line) compared to the prior clip. Since the bowel is closer on the prior clip we would be using that measurement instead of this one.

Real-time US for paracentesis
When the amount of fluid present in ascites is considered to be low, real time US can be used to drain or collect a sample of that fluid. Both linear and curved linear probes can be used for this procedure.

FA
FN

FV
Use of real time ultrasound for parecentesis. In line approach used in this clip. Notice the hyperchoic needle entering from the right
References.
1. Dancel R, Schnobrich D, Puri N, Franco-Sadud R, Cho J, Grikis L, Lucas BP, El-Barbary M; Society of Hospital Medicine Point of Care Ultrasound Task Force, Soni NJ. Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2018 Feb;13(2):126-135. doi: 10.12788/jhm.2940. PMID: 29377972.
2. Rodriguez Lima, D.R., Yepes, A.F., Birchenall Jiménez, C.I. et al. Real-time ultrasound-guided thoracentesis in the intensive care unit: prevalence of mechanical complications. Ultrasound J 12, 25 (2020). https://doi.org/10.1186/s13089-020-00172-9
3. Brogi E, Gargani L, Bignami E, et al. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit Care. 2017;21(1):325. Published 2017 Dec 28. doi:10.1186/s13054-017-1897-5
4. Cho J, Jensen TP, Reierson K, Mathews BK, Bhagra A, Franco-Sadud R, Grikis L, Mader M, Dancel R, Lucas BP; Society of Hospital Medicine Point-of-care Ultrasound Task Force, Soni NJ. Recommendations on the Use of Ultrasound Guidance for Adult Abdominal Paracentesis: A Position Statement of the Society of Hospital Medicine. J Hosp Med. 2019 Jan 2;14:E7-E15. doi: 10.12788/jhm.3095. PMID: 30604780; PMCID: PMC8021127.
5. Rudralingam V, Footitt C, Layton B. Ascites matters. Ultrasound. 2017;25(2):69-79. doi:10.1177/1742271X16680653
6. Szkodziak P, Czuczwar P, Pyra K, et al. Ascites Index - an attempt to objectify the assessment of ascites. J Ultrason. 2018;18(73):140-147. doi:10.15557/JoU.2018.0020.
7. Ennis, J. , Schultz, G. , Perera, P. , Williams, S. , Gharahbaghian, L. and Mandavia, D. (2014) Ultrasound for Detection of Ascites and for Guidance of the Paracentesis Procedure: Technique and Review of the Literature. International Journal of Clinical Medicine, 5, 1277-1293. doi: 10.4236/ijcm.2014.520163.


